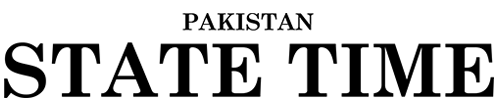Twelve diabetic patients in Pakistan have gone blind after receiving counterfeit Avastin, a cancer drug. The Drug Regulatory Authority of Pakistan (DRAP) has launched an investigation into the matter and has banned the distribution of three batches of Avastin.
Avastin is a drug that is approved in more than 130 countries to treat several types of cancer. It is also used off-label to treat diabetes-related eye conditions. In Pakistan, Avastin is not approved for any use in the eye.
The counterfeit Avastin that was given to the diabetic patients is believed to have been contaminated by a third party supplier. The counterfeit drug is thought to have contained harmful ingredients that caused the patients to lose their vision.
DRAP has banned the distribution of three batches of Avastin: H0353B11, B7266B20, and B7266B07. DRAP officials have also raided the facilities of GAPS, a pharmaceutical company in Lahore, and observed unhygienic conditions and non-sterile packaging.
The Pakistani government is investigating two local distributors of Roche’s Avastin cancer drug after 12 diabetic patients injected with the drug went blind. The Punjab Caretaker Health Minister has said that the police are questioning two men they believe to be the drug’s distributors in the province.
The sharp drop in the value of the local currency against the US dollar has inflated the price of drugs in Pakistan, many of which are either imported or based on imported ingredients. Record high inflation has also diminished the purchasing power of many people. This has led to an increase in the sale of counterfeit drugs in Pakistan.
Counterfeit drugs pose a serious health risk to patients because their content might be ineffective and contain harmful ingredients. Patients are advised to only purchase drugs from reputable pharmacies and to avoid purchasing drugs from online retailers or from unlicensed vendors.